 Lung Cancer Treatment
Lung Cancer Treatment
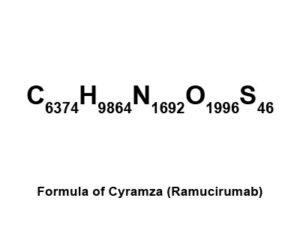
Cyramza (Ramucirumab) is an approved drug for the treatment of non-small cell lung cancer (NSCLC). It was approved by US FDA as a single agent for treatment of patients with advanced cancer. FDA granted review based on its potential to provide a significant improvement in safety or effectiveness in the treatment of cancer. It has also demonstrated the ability to extend the lives of patients and slow down the growth of tumor. FDA approved it to be used in combination with docetaxel for treatment of patients suffering from NSCLC.
Dosage
The approval of ramucirumab is based on the results of a large phase III trial called as REVEL. The recommended dosage is 10mg/kg IV Ramucirumab and 75 mg/m2 docetaxel. It should be administrated once every three weeks. It is available as an injection in single-dose vials. The medication works to block the development of new blood vessels. It is a type of treatment called as anti-angiogenic.
Precautions
Before you receive Ramucirumab, you should tell your doctor if you have high blood pressure or blood pressure problems or if you are planning to have surgery of any kind. If you are pregnant or may be pregnant, you should not take Cyramza as it may harm the unborn baby. Also, you should not breastfeed during the treatment. In case, you have had or are at high risk for heart attack, you must inform your healthcare practitioner. Tell your doctor about all the medications you are taking including prescription and OTC medicines.
Safety
Also, the safety as well as the effectiveness of Ramucirumab is different for everyone and individual results vary. It is always advisable to consult your health care professional and talk about what Ramucirumab in combination with docetaxel can work for your treatment.
Don’t forget to pen down your thoughts on the rise of lung cancer in the comments section below.
The information offered in this blog is for educational purposes only and is not a substitute for medical advice. Consult your doctor before intake of any medication or treatment.
